If we bring two electric charges close to each other, they exert a force on each other. This force is known as the electrostatic or electric force. It is a natural property of electric charges. Every electric charge or charged body exerts an electric force on another charged body near it. In this article, I’m going to discuss electrostatic force, its equation, properties and examples.
What is Electrostatic Force?
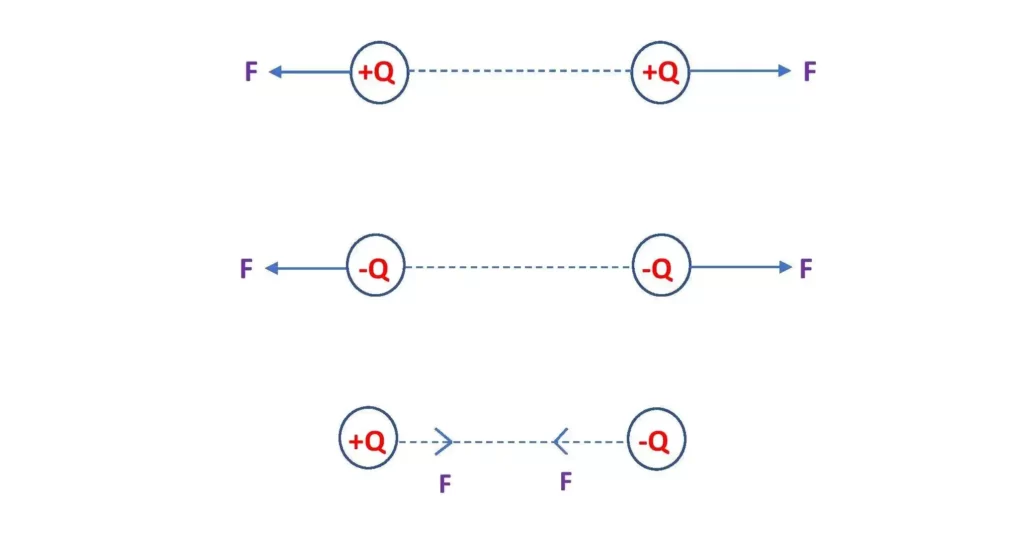
The electrostatic or electric force is defined as the force between two electric charges when one of these is placed inside the electric field of the other. If we say a charge q is placed in the electric field of another charge Q, then Q is the source charge and q is the test charge. So, the electric force is the force between a source charge and a test charge.
Formula of electrostatic force
Let a source charge Q creates an electric field E around it. If we bring a test charge q in this field region, the electric force on the charge q is, \small {\color{Blue} F=qE}…….(1)
Sometimes, it is called Lorentz force equation in electrostatics.
Again, the equation of electric field at r distance from a source charge Q is \small {\color{Blue} E=\frac{1}{4\pi \epsilon _{0}}\frac{Q}{r^{2}}}.
Where \small {\color{Blue} \epsilon _{0}} is the permittivity in free space.
Then the equation for electrostatic force between two point charges Q and q separated by a distance r is, \small {\color{Blue} F=\frac{1}{4\pi \epsilon _{0}}\frac{Qq}{r^{2}}}…….(2)
This is nothing but the equation of Coulomb’s Law of electrostatics. Actually, Coulomb’s law gives the equation of electric force between electric charges.
Properties of electrostatic force
- Electrostatic force is a Conservative force.
- This force acts between the electric charges.
- Electric force may be attractive or repulsive.
- This force obeys the inverse square law of the distance between the charges. The magnitude of this force decreases with an increase in distance between the charges.
- Coulomb’s Electrostatic force depends on the medium. The magnitude of electric force for the same two charges at the same distance is different for different mediums.
- The electric force is not applicable for the charges placed at a distance smaller than the nuclear distance.
Examples of electric force in daily life
There are some interesting examples of electrostatic forces that we observe in some situations. These are listed below –
- Rubbing of dry hair with a comb in the winter season charges the comb and therefore, the comb will attract small pieces of paper.
- You can feel the electrostatic force near the screen of a TV if you bring your hand near the TV screen. When a TV is ON, it charges the dust particles near the screen and then attracts the dust on the screen. Therefore, a layer of dust can be seen in a few minutes after cleaning the previous layer.
- Rubbing Silk or woolen dresses with a dry plastic chair can electrify the dresses and they can offer electric force to small pieces of paper.
- A photograph sticks to a wrapper due to this force.
Comparison between Coulomb’s electric force and Gravitational force
There are some similarities and also some differences between electrostatic force and Gravitational force. Here we are going to discuss those.
Similarities between Electrostatic and Gravitational forces
- Both Electric and Gravitational force are the conservative forces.
- They obey the inverse square law of distance.
Differences between Electrostatic force and Gravitational force
- Electrostatic force may be attractive or repulsive. But Gravitational force is always attractive in nature.
- Coulomb’s Electrostatic force between the charges depends on the medium where the charges are placed. But the Gravitational force is independent of the medium.
- The electric force is much stronger than the Gravitational force.
This is all from this article on the electric force between electric charges. If you still have any doubts on this topic you can ask me in the comment section.
Thank you!
Related posts: