Impurity mixed semiconductors are known as the extrinsic semiconductors. Now, there are two types of extrinsic semiconductors. These are n type and p type semiconductors. They have different properties and the ways of formation are also different. We already have discussed p type semiconductors in another article. In this article, we are going to discuss the n type semiconductor material, its formation, properties, examples and uses.
Formation of n type semiconductor
A pure semiconductor like silicon, Germanium, etc. has four valence electrons. In a pure semiconductor crystal, each atom produces four covalent bonds with four neighbor atoms in the crystal. Now, to produce a n-type semiconductor, pentavalent impure atoms like Arsenic (As), Phosphorus (P), etc. are needed to be doped in the pure semiconductor crystal. The doping concentration is very less (only one impure atom per one lakh pure Silicon or Germanium atoms).
Suppose, impure pentavalent atoms are doped in pure silicon crystal. After the doping, each pentavalent atom produces four covalent bonds with four nearest neighbor atoms of silicon. Now, the pentavalent atom has another valence electron which cannot find any other neighbor silicon atom to create another covalent bond. So, an extra free electron remains freely in the semiconductor crystal. This extra free electron increases conductivity of the semiconductor crystal. Now, there are thousand of impure atoms in the crystal. Each impure atom will give unpaired free electron. As a result, the amount free electrons becomes high and thereby, the conductivity of the semiconductor crystal increases significantly even at room temperature.
In this way one can produce n-type semiconductors to increase the overall conductivity.
Properties of n type semiconductor
Followings are the properties of n-type semiconductors-
- This type of semiconductors form due to the doping pentavalent atoms in pure semiconductor crystal.
- The majority carriers in n-type semiconductors are free electrons.
- Minority carriers of this type semiconductors are holes.
Examples of n type semiconductors
Phosphorus doped silicon, Arsenic doped silicon, Phosphorus doped Germanium, Arsenic doped Germanium, etc. are the examples of n-type semiconductors. Any other semiconductors with pentavalent impurities are n-type semiconductors.
Energy band diagram for n-type semiconductor
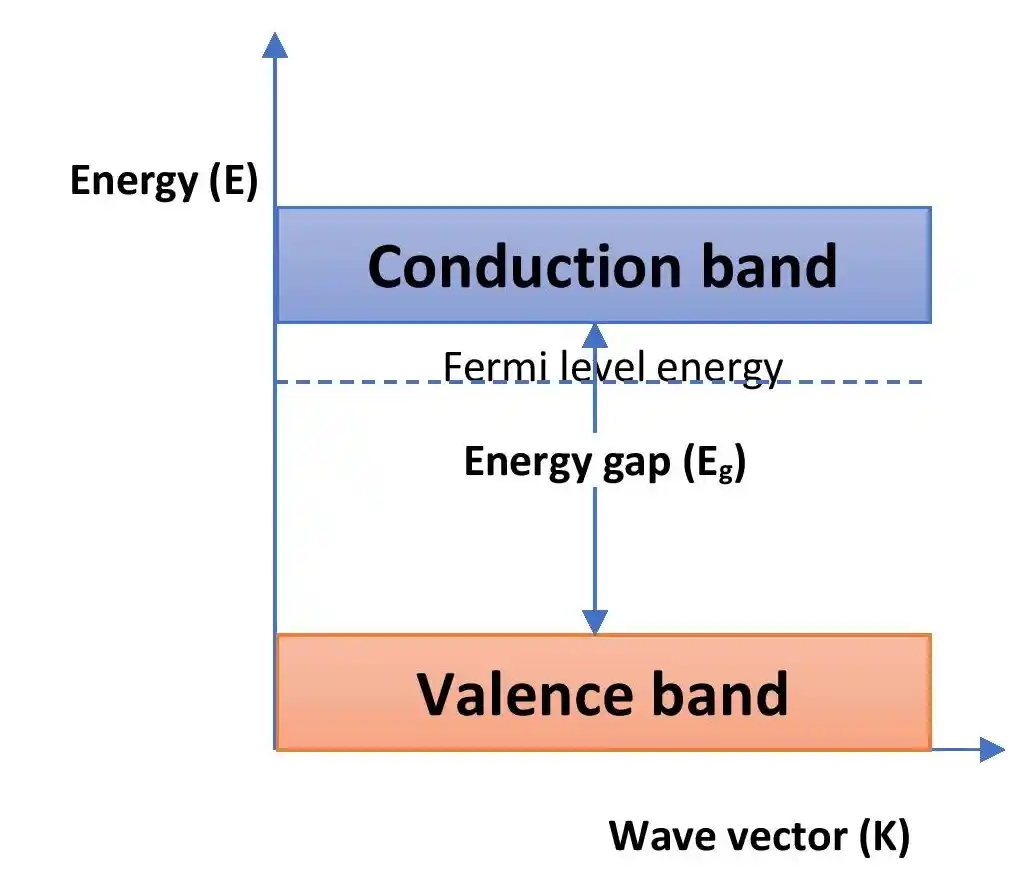
The above figure shows the energy band diagram of n-type semiconductors. For this type of semiconductors, the Fermi level shifts towards the conduction band. Because, the density of free electrons becomes higher for n-type semiconductor than the intrinsic one.
Advantages of n-type semiconductors
A pure semiconductor material has poor conductivity. But extrinsic semiconductors have much higher conductivity even at room temperature. So, the n-type semiconductors are useful in room temperature as well. Again, this type of semiconductor has free electrons as the majority carriers which allows the semiconductor material to behave like a traditional conductor at a fixed temperature.
This is all from this article. If you have any doubt on this topic, you can ask me in the comment section. Thank you!
Related posts:
- p-type semiconductor
- Intrinsic and extrinsic semiconductors
- Holes and their origin
- Why do we need doping in semiconductors?
Comments are closed.